Once patients are diagnosed with cancer, they begin their journey by forming new relationships with a multidisciplinary healthcare team that will support them during various points along the way. The definition of the patient journey has been broadened to answer important questions on how patients enter the healthcare system. For example, “Was the patient identified through a screening program, primary care setting, or emergency room?” and “How was the patient navigated to the practice?” From point of entry into the system, Utah Cancer Specialists (UCS) established a protocol to follow patients and assessed, during key points along their cancer journey, whether the best clinical decisions were made to achieve optimal clinical outcomes. This was accomplished through establishing and maintaining strong relationships and clear communication with the multidisciplinary care team whose members do not always work in the same location and who oftentimes do not have access to other data sources that further document the patient’s cancer journey.
UCS is a community-based practice that offers numerous healthcare services, allowing patients to be treated close to home. The success of this practice is based on creating a paradigm for oncology care that involves a stakeholder ecosystem consisting of physicians, surgeons, radiologists, diagnosticians, pharmacists, nurses, and patients.
As part of a commitment to excellence, and as a pillar of success, it was necessary for this practice to stay abreast of advances in technology and targeted treatment options in a continually evolving landscape. Precision medicine became a key component in guiding the treatment journey of its patients.
Addressing the Challenges of Precision Medicine
Once an individual with cancer is diagnosed and is staged, the next step is to assess whether molecular testing should be performed, based on National Comprehensive Cancer Network (NCCN) guidelines. The results of the genomic test are used to select an appropriate treatment regimen. Although this process may seem straightforward, there exists an intricate choreography that occurs between the multidisciplinary team and the underlying technology used by each team member. The factors to consider when determining if a test can be performed are: when tests are ordered; how the tissue sample was processed; and the quantity and quality of the tissue. UCS developed a standard tissue processing protocol with the multidisciplinary team to provide optimal molecular pathology testing. Tissue collection and curation requirements not only affect whether a molecular test can be ordered; they also impact patient eligibility and enrollment in clinical trials that have strict tissue requirements.
Advances in precision medicine have opened the door to various enhancements in molecular testing capabilities, from next-generation sequencing-based gene panels to liquid biopsy. The increased number of new tests available coupled with the large volume of novel treatment options have contributed to advances in precision medicine but have also exposed the increasing challenge of coverage and reimbursement. The numerous options for testing and treatment have financial implications for both patients and practices.
Patients are often responsible for out-of-pocket costs associated with testing that has yet to be supported by NCCN guidelines. Advances have been made to Medicare coverage that allow certain tests to be covered by Medicare beneficiaries diagnosed with advanced, recurrent, or late-stage cancer.1 In some instances, the burden of providing additional clinical support is placed on the laboratories performing the tests in order for them to be reimbursed. These challenges must be taken into consideration, along with investments in information technology infrastructure, scaling, and clinical trials programs.
Using Data and Analytics to Improve Clinical Outcomes
Creating an integrated network based on the multidisciplinary team paradigm allows for a unique and comprehensive understanding of the cancer population. For example, how many patients with metastatic non–small-cell lung cancer tested positive for EGFR and, as a result, were prescribed a tyrosine kinase inhibitor? Of the patients who were initially placed on systemic therapy and later moved over to a tyrosine kinase inhibitor, what was the turnaround time for the test results?
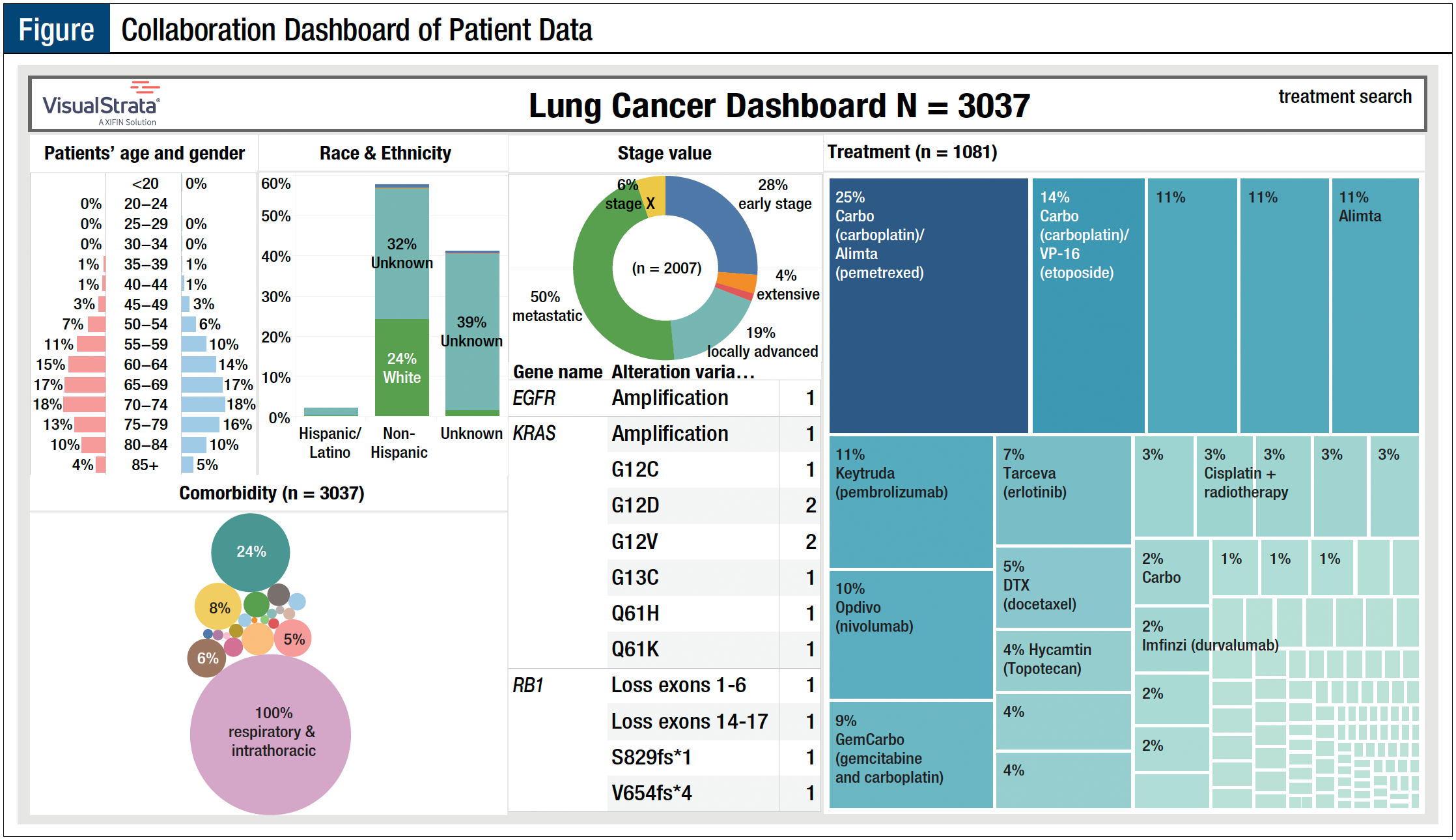
The Figure shows how UCS was able to identify and report on these indicators and identify important gaps in knowledge. For example, “How many patients with lung cancer were tested, and of those tested, how many had a positive result?” and “Were all testing options made available to obtain the best clinical outcome?” Some of these gaps in knowledge helped to shed light on treatment prescribing patterns and closed the loop in a complex system with several moving parts whose collective objective was to ensure the best clinical outcome for patients diagnosed with cancer.
Given the inherent challenges, integrating the community oncology care team workflow and connecting the disparate systems in a meaningful manner is essential to the success of the precision medicine approach. Early on, UCS was committed to research that would help to improve clinical outcomes. Data aggregation and curation across unlinked systems in a longitudinal approach to classify individuals into subpopulations that differ in their susceptibility to a particular disease, or their response to a specific treatment, became a priority for the practice. Data and analytics are strategic initiatives that provided visibility into the entire patient journey. Linking disparate data sources together (ie, diagnostics and financials) allowed UCS the ability to analyze, track, and forecast costs of treating patients across regions, and extrapolate the course of future events and data-driven risk stratification.
Reference
- Centers for Medicare & Medicaid Services. National coverage determination (NCD) for next generation sequencing (NGS) (90.2). Updated March 2021. www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=372. Accessed April 17, 2021.