Introduction
Data aggregation and management challenges for community oncology practices are becoming more complicated with the advent and application of precision medicine. As advances are made in clinical molecular technologies, and the volume, velocity, and value of this data increases, adjustments must be made to incorporate informatics approaches to inform data-driven decision-making through robust and reliable integration of multiple heterogeneous data sources into one consolidated analytics platform.
Historically, the focus and associated applications have been solely comprised of data stored in structured fields. However, in the oncology universe, traditional electronic medical records do not capture all the disparate and detailed information related to a patient’s specific oncology case. Key clinical decision-making information is often stored in varying formats, including portable document formats (PDFs), Digital Imaging and Communications in Medicine (DICOM) images, clinical notes, and potentially other unstructured formats, which may not be consumed or reflected in the subsequent analysis.
A purpose-built technology solution with a data-driven informatics framework that enables information from disparate sources to be synthesized and integrated is imperative. This technology solution must be flexible and capable of consuming various types of data provided from labs, embedded within PDFs, standardized imaging, and clinical notes into a unified, knowledge-based, enabled registry. Organized and accessible information facilitates the ability to view the longitudinal disease journey and isolate subpopulations to help evaluate specific questions of interest.
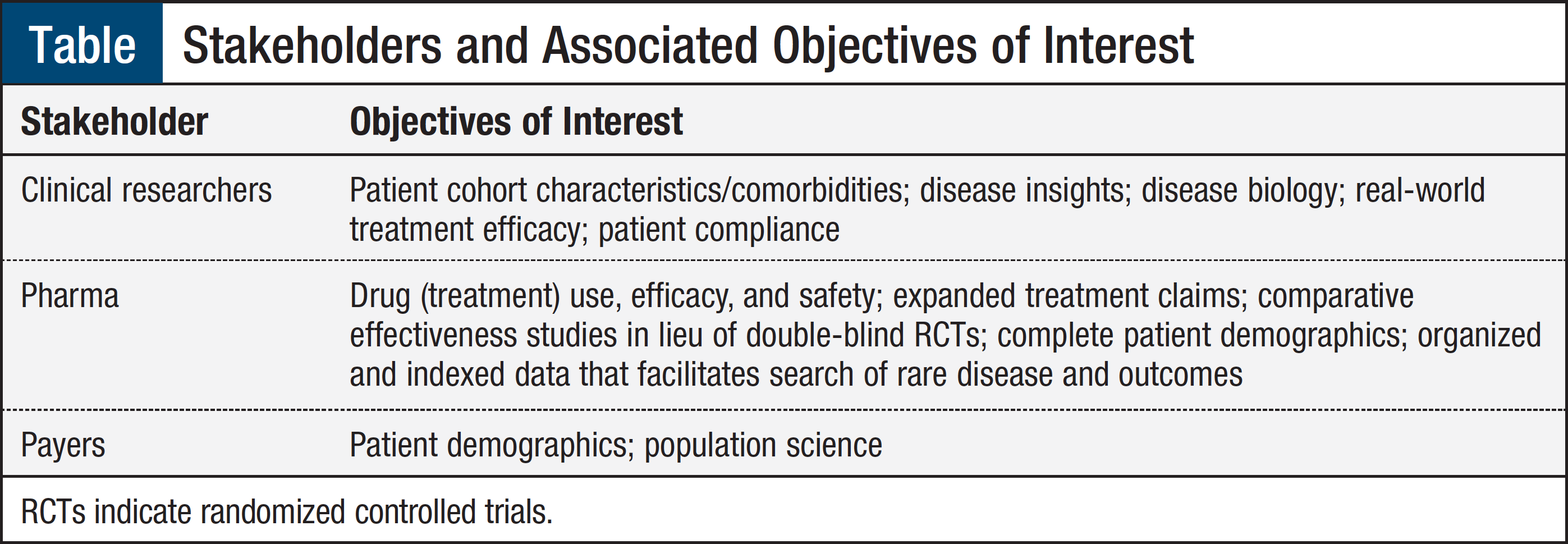
The focus of this article is to outline the design of a real-time registry that aggregates heterogeneous clinical and genomic data (along with other molecular results) in a unified structure. Data turned into meaningful information can be used to identify, vet, and fulfill requirements to support multiple research projects, clinical trials, clinical quality improvement programs, and real-world evidence initiatives. Rule-based approaches common in healthcare informatics can be applied to data curation that can be used for different objectives as shown in the Table.
How a Single Source of Well-Organized Data Enables Real-World Data Analytics in Precision Oncology
The following use cases illustrate the reuse of analyzed data.
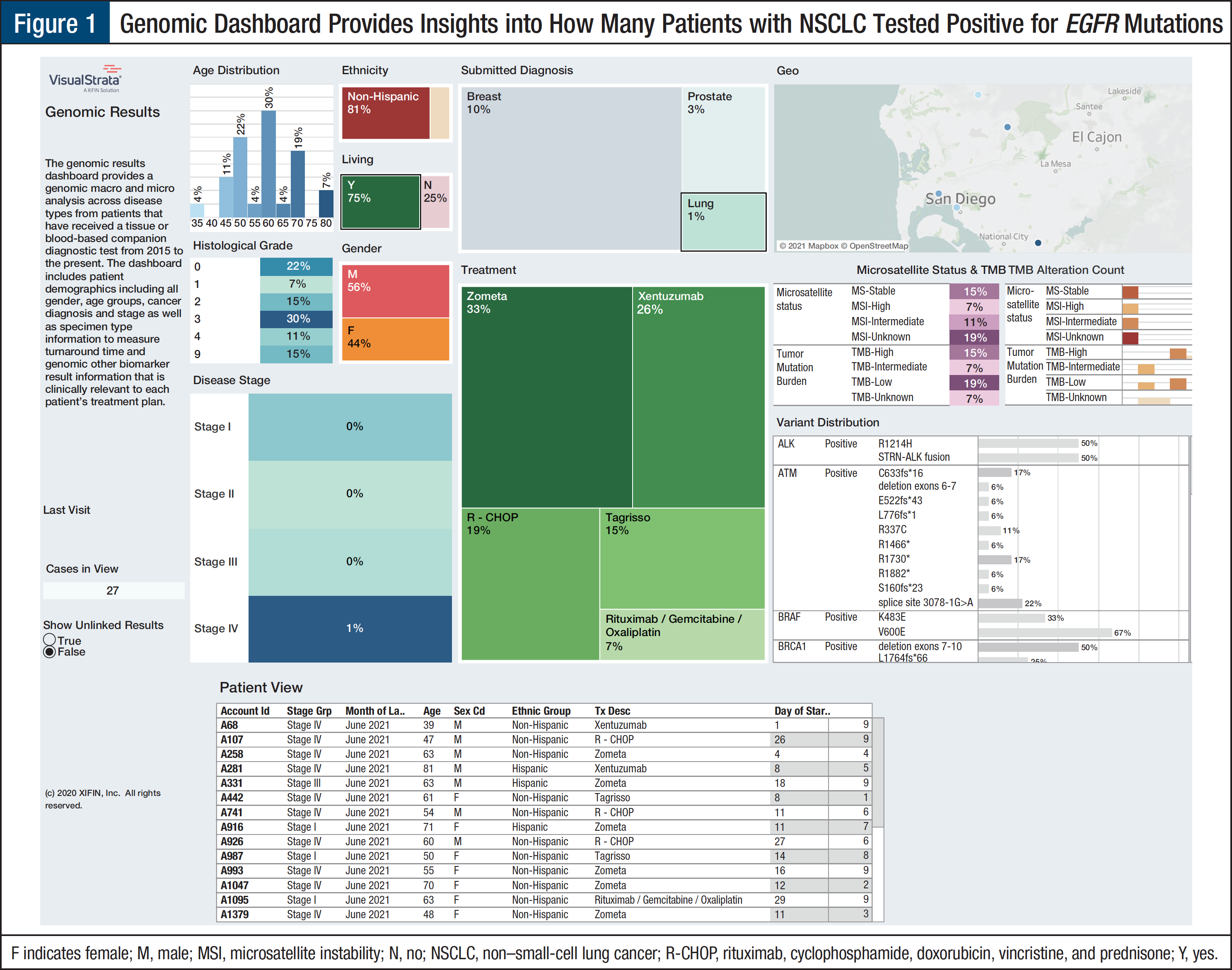
Use Case 1: Identification of NSCLC Cohort of Clinically Relevant Mutations by American Joint Committee on Cancer Stage1
An integrated platform can provide insight into the number of genomic test results for each cancer type by stage of disease at diagnosis. The National Comprehensive Cancer Network (NCCN) guidelines recommend testing for EGFR mutations in patients with metastatic non–small-cell lung cancer (NSCLC) as well as in patients with early-stage NSCLC whose cancer is resectable.2 The dashboard can provide insight into clinician compliance with NCCN guidelines to optimize outcomes for their patients. Except for KRAS, which is a prognostic marker and predicts nonresponse to tyrosine kinase inhibitors in patients with EGFR mutations, the molecular genomic biomarkers identify patients who are eligible for targeted therapies (Figure 1). These types of results are of major interest to pharmaceutical researchers and payers.
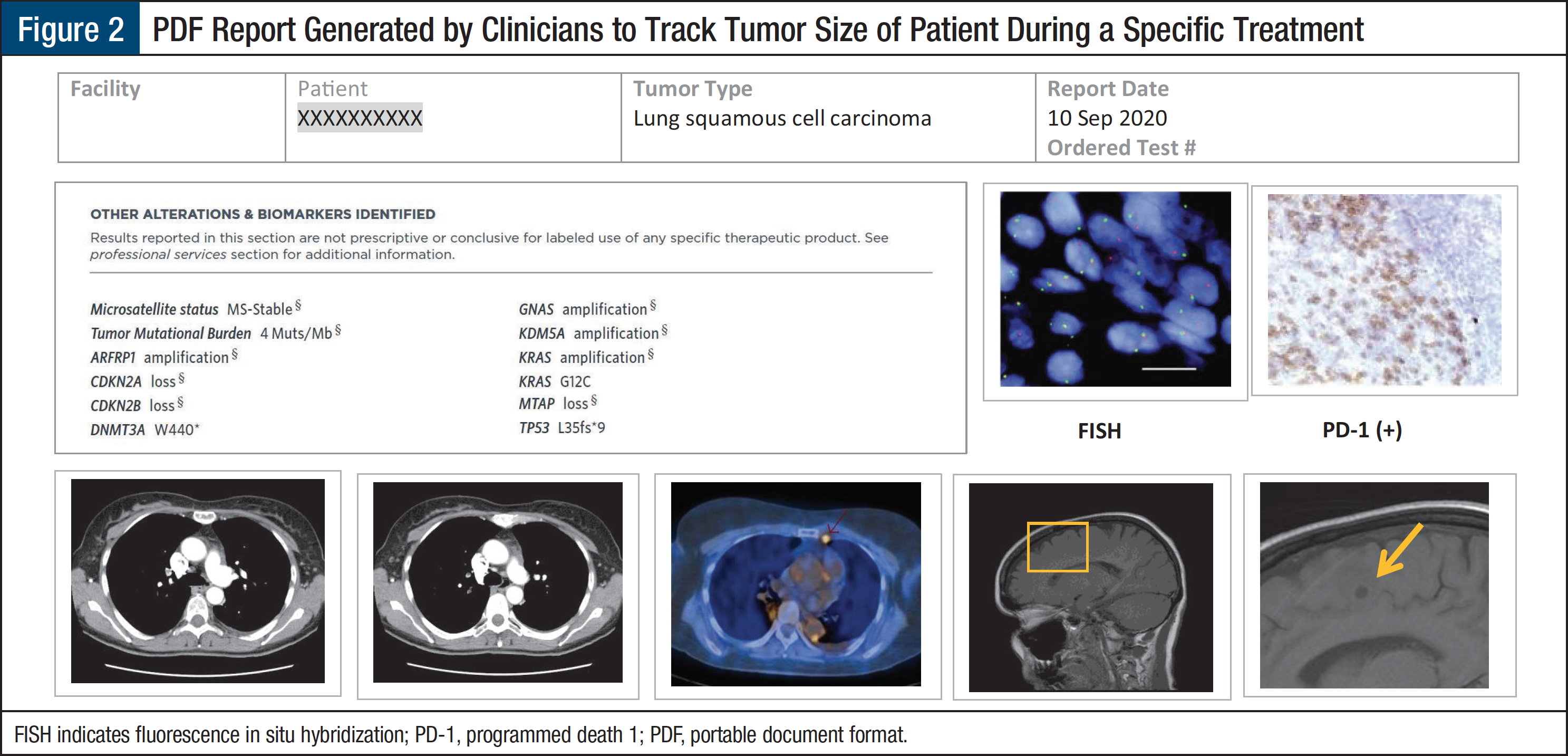
Use Case 2: Evaluation and Response Rates of Patients with NSCLC
Based on Use Case 1, we can take this a step further to evaluate the response rates of patients treated with osimertinib (Tagrisso) who were diagnosed with NSCLC by stage. Tumor size is evaluated at baseline prior to initiation of treatment and at scheduled intervals throughout and at the conclusion of the therapy. The information related to tumor size leverages the rich content in the clinical notes in addition to DICOM imaging.3 Combined imaging and diagnostic information is critical to determine response rates for any given patient receiving treatment. The approach to combine all content can be provided in a report (Figure 2). The dashboards and reports provide meaningful information and insights to pharmaceutical researchers, clinical researchers, and payers.
Conclusion
It is important to eliminate any barriers that could impede the optimization of care for patients with cancer. Within the current oncology healthcare model, one such challenge is the storage of disparate information in various data sources and data formats. Harmonizing this information in one comprehensive platform provides insights into specific questions of interest and helps enhance clinical outcomes through providing continuity and transparency into the convoluted patient journey. These dashboards help to underscore areas of interest that can be explored further and used to change processes or highlight opportunities to educate providers.
References
- American Joint Committee on Cancer. Cancer staging system. www.facs.org/quality-programs/cancer/ajcc/cancer-staging. Accessed June 17, 2021.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Non-Small Cell Lung Cancer. Version 5.2021. June 15, 2021. www.nccn.org/professional/physician_gls/pdf/nscl.pdf. Accessed June 28, 2021.
- Digital Imaging and Communications in Medicine. Current edition. www.dicomstandard.org/current. Accessed June 17, 2021.